Multiple Choice
Suppose that a certain atom possesses only four distinct energy levels. Assuming that all transitions between levels are possible, how many spectral lines will this atom exhibit? 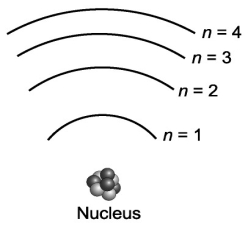
A) three
B) four
C) six
D) unlimited
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: What is the approximate mass of a
Q12: Why are the atomic masses listed in
Q13: The element bromine,Br (atomic number 35),has two
Q26: Which of the following might best explain
Q50: If an element has 10 protons and
Q86: About how many times larger is a
Q87: Which of the following atoms is the
Q88: How is it possible for an atom
Q92: If the particles of a cathode ray
Q93: In the following diagram, which of the