Multiple Choice
Consider the various frequencies of the three photons emitted from the following three individual electron transitions in the figure below: n=3 to n=2; n=2 to n=1; n=3 to n=1. These transitions would produce three spectral lines in a spectroscope. If the energy spacing between the levels were equal, would this affect the number of spectral lines? 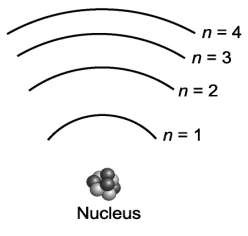
A) Yes, two otherwise separate lines would converge into a single more intense line.
B) No, but the spacing between the spectral lines would change.
C) Yes, two otherwise separate lines would converge into a single less intense line.
D) No, but some would then require a prism in order to be seen.
Correct Answer:

Verified
Correct Answer:
Verified
Q10: The _ represents the complete range of
Q11: List the following atoms in order of
Q13: How might you distinguish a sodium-vapor street
Q14: Rank the following by relative frequency from
Q16: From which of the following atoms would
Q17: Which of the following concepts underlies all
Q20: Isotopes of the same element have a
Q22: What do the components of a conceptual
Q25: Which contributes more to an atom's mass:
Q80: The mass number of an element is<br>A)the