Multiple Choice
Why does water not freeze at 0°C when either ions or molecules other than 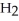 O are present?
O are present?
A) Molecules or ions dissolved in the water give the water molecules something else to easily bond with, thus allowing the solution to freeze well above 0°C.
B) Molecules or ions dissolved in the water increase the number of water molecules at the solid-liquid interface, thus allowing for the rate of ice formation to increase at a temperature above 0°C.
C) When molecules or ions are dissolved in the water, these solute molecules both take up space and inhibit the formation of the solid phase, thus lowering the freezing point temperature below 0°C.
D) Molecules or ions dissolved in the water will sometimes raise the freezing point above 0°C and other times lower it below 0°C depending on the nature of these solute particles.
Correct Answer:

Verified
Correct Answer:
Verified
Q112: Why does evaporation of a liquid also
Q113: Given the following specific heats and that
Q114: Would you expect the surface tension of
Q115: Which of the following statements does not
Q116: Heat is absorbed by a substance when
Q118: From the diagram above describing the phase
Q119: If all of the capillaries pictured above
Q120: How much heat is required to raise
Q121: A cohesive force is best described as
Q122: Which of the following statements best describes