Multiple Choice
Why is calcium chloride, 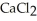 , more effective at melting ice than sodium chloride, NaCl?
, more effective at melting ice than sodium chloride, NaCl?
A) Calcium ions are both larger and more highly charged and thus more effective in breaking the ice crystal structure.
B) Calcium ions are smaller than sodium ions and thus more able to get in the way of the water molecules attempting to form the solid state lattice.
C) Calcium chloride produces three ion particles while sodium chloride produces only two. Greater numbers of ions are more effective at decreasing the number of water molecules entering the solid phase.
D) Sodium ion is a natural water softener and therefore not as effective in inhibiting the formation of the solid state of water from the liquid.
Correct Answer:

Verified
Correct Answer:
Verified
Q34: The specific heat of a substance is
Q35: If it takes 200 J to raise
Q36: What happens to the freezing temperature of
Q37: Evaporation is _.<br>A)always a cooling process<br>B)sometimes a
Q38: Why does water have a high specific
Q40: What happens to the temperature of something
Q41: From the diagram above describing the phase
Q42: To impart a hickory flavor to a
Q43: How does the combined volume of the
Q44: Would fevers run higher or lower if