Multiple Choice
Which is the stronger acid: H-F or H-I?
A) H-F, because the electronegativity of fluorine is higher than that of iodine making the H-F bond more polar and more likely to release the hydrogen ion to solution.
B) H-F, because acid strength for binary acids decreases "down the group" of the periodic table.
C) H-I, because iodine releases the hydrogen in order to form 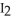 (s) at room temperature.
(s) at room temperature.
D) H-I, because the H-I bond is weaker and so it is easier to break to form hydrogen ions.
Correct Answer:

Verified
Correct Answer:
Verified
Q5: What would the concentration of OH<sup>-</sup> be
Q94: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6597/.jpg" alt=" -Which of the
Q98: What atom in the hydronium ion, H<sub>3</sub>O⁺,
Q99: Carbon dioxide reacts with water to form
Q100: Qualitatively, what happens to the hydroxide ion
Q105: If you were to increase the pH
Q106: Adding more carbon dioxide to the atmosphere
Q107: If the rate at which we produce
Q140: For the following reaction,identify whether the compound
Q142: For the following acid-base reaction,identify which salt