Multiple Choice
When a hydronium ion concentration equals 1 × 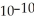 moles per liter, what is the pH of the solution? Is the solution acidic or basic?
moles per liter, what is the pH of the solution? Is the solution acidic or basic?
A) The pH of this solution is 4, which is acidic.
B) The pH of this solution is 8, which is basic.
C) The pH of this solution is 6, which is acidic.
D) The pH of this solution is 10, which is basic.
Correct Answer:

Verified
Correct Answer:
Verified
Q27: If the pH of a solution is
Q49: The overall rate at which humans are
Q50: In the following buffer system, what happens
Q52: Which of the following items would you
Q55: What would the concentration of H<sub>3</sub>O<sup>+</sup> be
Q56: How might warmer oceans accelerate global warming?<br>A)The
Q58: Arrange the following images of an aqueous
Q59: How might you tell whether or not
Q77: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6597/.jpg" alt=" -Which of the
Q87: In the reaction below,what does the symbol