Multiple Choice
When a hydronium ion concentration equals 1 × 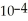 moles per liter, what is the pH of the solution? Is the solution acidic or basic?
moles per liter, what is the pH of the solution? Is the solution acidic or basic?
A) The pH is of this solution is 4 and it is acidic.
B) The pH is of this solution is 8 and it is basic.
C) The pH is of this solution is 6 and it is acidic.
D) The pH is of this solution is 10 and it is basic.
Correct Answer:

Verified
Correct Answer:
Verified
Q20: What is the relationship between the hydroxide
Q46: What is the main characteristic of a
Q58: Why is carbon dioxide able to be
Q79: Which of the following items would you
Q80: If you had a 1 M solution
Q83: If you had a 1 M solution
Q87: Which of the following statements about strong
Q128: How do you make a proton out
Q150: What happens to the corrosive properties of
Q153: Which of the following statements about strong