Multiple Choice
Explain why caprylic acid, C 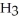 (C
(C 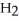
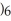 COOH, dissolves in a 5 percent aqueous solution of sodium hydroxide but caprylaldehyde, C
COOH, dissolves in a 5 percent aqueous solution of sodium hydroxide but caprylaldehyde, C  (C
(C 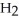
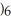 CHO, does not.
CHO, does not.
A) With two oxygens the caprylic acid is about twice as polar the caprylaldehyde.
B) The caprylaldehyde is a gas at room temperature.
C) The caprylaldehyde behaves as a reducing agent, which neutralizes the sodium hydroxide.
D) The caprylic acid reacts to form the water soluble salt.
Correct Answer:

Verified
Correct Answer:
Verified
Q11: If an alcoholic beverage is 20 percent
Q40: What is meant by the term octane
Q41: Which of the following molecules could be
Q42: The phosphoric acid salt of caffeine has
Q44: Organic synthesis is useful _.<br>A)as an intellectual
Q46: If you saw the label "phenylephrine HCl"
Q47: An organic compound is any molecule _.<br>A)with
Q49: Which of the following is the simplest
Q54: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6597/.jpg" alt=" -Which of the
Q94: What is a functional group?<br>A)a set of