Multiple Choice
When aqueous solutions of AgNO3 and KI are mixed, AgI precipitates. The balanced net ionic equation is ________.
A) Ag+ (aq) + 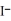 (aq) → AgI (s)
(aq) → AgI (s)
B) Ag+ (aq) + NO3- (aq) → AgNO3 (s)
C) Ag+ (aq) + NO3- (aq) → AgNO3 (aq)
D) AgNO3 (aq) + KI (aq) → AgI (s) + KNO3 (aq)
E) AgNO3 (aq) + KI (aq) → AgI (aq) + KNO3 (s)
Correct Answer:

Verified
Correct Answer:
Verified
Q1: In which reaction does the oxidation number
Q65: Which one of the following is a
Q84: The net ionic equation for the dissolution
Q101: A neutralization reaction between an acid and
Q110: A stock solution of HNO<sub>3</sub> is prepared
Q114: The molarity (M)of an aqueous solution containing
Q117: There are _ mol of bromide ions
Q118: A tenfold dilution of a sample solution
Q121: How many grams of sodium chloride are
Q122: Silver ions can be precipitated from aqueous