Multiple Choice
The molarity (M) of an aqueous solution containing 22.5 g of sucrose (C12H22O11) in 35.5 mL of solution is ________.
A) 0.0657
B) 1.85 × 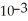
C) 1.85
D) 3.52
E) 0.104
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Which of the following are weak electrolytes?<br>HNO<sub>3</sub><br>HF<br>NH<sub>3</sub><br>LiBr<br>A)HNO<sub>3</sub>,
Q35: How many moles of BaCl<sub>2</sub> are formed
Q37: A titration reached the equivalence point when
Q38: The point in a titration at which
Q39: With which ion will the mercury ion
Q41: What mass (g)of barium iodide is contained
Q42: Which solution has the same number of
Q43: Which of these metals will be oxidized
Q43: Calculate the number of grams of solute
Q78: The molarity of a solution prepared by