Multiple Choice
Which electron configuration represents a violation of the Pauli exclusion principle?
A) 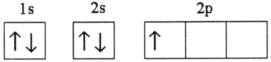
B) 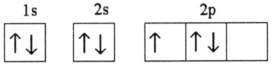
C) 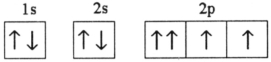
D) 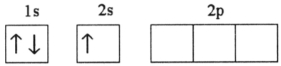
E) 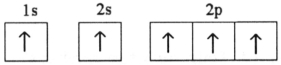
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q34: In a p<sub>x</sub> orbital,the subscript x denotes
Q62: The energy (J)required for an electronic transition
Q85: What color of visible light has the
Q99: The energy of a photon that has
Q104: What wavelengths correspond to the visible region
Q108: The de Broglie wavelength of a car
Q111: The larger the principal quantum number of
Q127: How many p-orbitals are occupied in a
Q145: When the electron in a hydrogen atom
Q186: Which one of the following represents an