Multiple Choice
Which one of the following is the correct electron configuration for a ground-state nitrogen atom?
A) 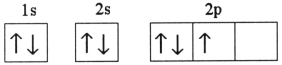
B) 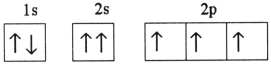
C) 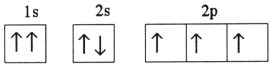
D) 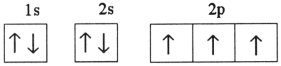
E) None of the above is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: The angular momentum quantum number for the
Q26: Using Bohr's equation for the energy levels
Q27: Electromagnetic radiation with a wavelength of 425
Q45: An electron cannot have the quantum numbers
Q87: A mole of yellow photons of wavelength
Q112: The n = 2 to n =
Q132: Which of the subshells below do not
Q156: The ground state electron configuration of copper
Q167: Ham radio operators often broadcast on the
Q176: The principal quantum number of the first