Multiple Choice
Which electron configuration denotes an atom in its ground state?
A) 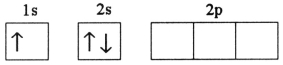
B) 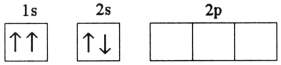
C) 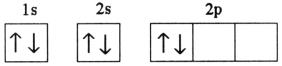
D) 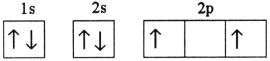
E) 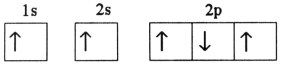
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: What is the wavelength of light (nm)that
Q60: The square of Schrodinger's wave equation is
Q88: _-orbitals are spherically symmetrical.<br>A)s<br>B)p<br>C)d<br>D)f<br>E)g
Q98: The frequency of a photon that has
Q113: Which of the subshells below do not
Q149: The energy of a photon that has
Q156: The de Broglie wavelength of an electron
Q157: The correct ground-state electron configuration for molybdenum
Q159: An NMR spectrum results from photon irradiation
Q163: The correct ground-state electron configuration for silver