Multiple Choice
Which electron configuration represents a violation of Hund's rule for an atom in its ground state?
A) 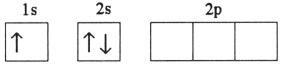
B) 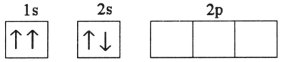
C) 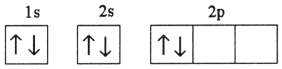
D) 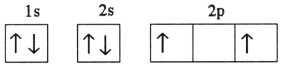
E) 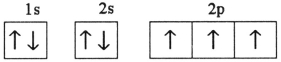
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: Which quantum numbers must be the same
Q37: Electromagnetic radiation with a wavelength of 640
Q38: What is the de Broglie wavelength (m)of
Q77: Elements in group _ have a np<sup>6</sup>
Q82: The de Broglie wavelength of an electron
Q90: Which of the following is not a
Q118: How many quantum numbers are necessary to
Q137: Which quantum number determines the energy of
Q157: Which one of the following orbitals can
Q181: The wavelength of light that has a