Multiple Choice
Which of the following correctly represents the second ionization of aluminum?
A) Al+ (g) + e- → Al (g)
B) Al (g) → 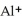 (g) + e-
(g) + e-
C) Al- (g) + e- → Al2- (g)
D) Al+ (g) + e- → Al2+ (g)
E) Al+ (g) → Al2+ (g) + e-
Correct Answer:

Verified
Correct Answer:
Verified
Q17: A group of ions all containing the
Q29: The substance _ is always produced when
Q36: Alkaline earth metals _.<br>A)have the smallest atomic
Q37: Which nonmetal exists as a diatomic solid?<br>A)bromine<br>B)antimony<br>C)phosphorus<br>D)iodine<br>E)boron
Q48: The first ionization energies of the elements
Q76: [Kr]5s<sup>2</sup> is the electron configuration for _.
Q117: The most common sulfur ion has a
Q118: The degree of interaction between two electrical
Q119: Metals can be _ at room temperature.<br>A)liquid
Q127: When two elements combine to form a