Multiple Choice
The formal charge on carbon in the molecule below is ________. 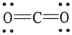
A) 0
B) +1
C) +2
D) +3
E) -1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: Determining lattice energy from Born-Haber cycle data
Q44: In the molecule below,which atom has the
Q52: Of the following,_ cannot accommodate more than
Q66: Elements from opposite sides of the periodic
Q87: In a reaction, if the bonds in
Q90: The Lewis structure of the CO<sub>3</sub><sup>2-</sup> ion
Q90: The ability of an atom in a
Q93: The principal quantum number of the electrons
Q106: What species has the electron configuration [Ar]3d<sup>4</sup>?<br>A)Mn<sup>2+</sup><br>B)Cr<sup>2+</sup><br>C)V<sup>3+</sup><br>D)Fe<sup>3+</sup><br>E)K<sup>+</sup>
Q127: When a metal gains an electron,the process