Multiple Choice
The formal charge on sulfur in SO42- is ________, where the Lewis structure of the ion is: 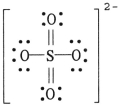
A) -2
B) 0
C) +2
D) +4
E) -4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: Using the table of average bond energies
Q23: There are _ valence electrons in the
Q24: What is the electron configuration for the
Q27: From the information given below, calculate the
Q29: Using the noble gas shorthand notation, write
Q86: Based on the octet rule,boron will most
Q87: As electronegativity difference increases,bond length will decrease.
Q88: How many equivalent resonance structures can be
Q111: The electron configuration of the phosphide ion
Q133: The ion ICl<sub>4</sub><sup>-</sup> has _ valence electrons.<br>A)34<br>B)35<br>C)36<br>D)28<br>E)8