Multiple Choice
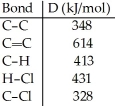
Using the table of bond dissociation energies, the ΔH for the following gas-phase reaction is ________ kJ. 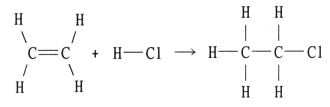
A) -44
B) 38
C) 304
D) 2134
E) -38
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: Which of the following would have to
Q23: A nonpolar bond will form between two
Q43: Bond enthalpy can be positive or negative.
Q46: A valid Lewis structure of _ cannot
Q49: Using the table of average bond energies
Q50: Which energy change corresponds to the first
Q51: The central atom in _ does not
Q68: For a given arrangement of ions,the lattice
Q85: Bond enthalpy is _.<br>A)always positive<br>B)always negative<br>C)sometimes positive,
Q130: Which of the following would have to