Multiple Choice
The molecular geometry of the right-most carbon in the molecule below is ________. 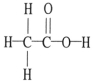
A) trigonal planar
B) trigonal bipyramidal
C) tetrahedral
D) octahedral
E) T-shaped
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: The central atom in the O<sub>3</sub> molecule
Q30: In comparing the same two atoms bonded
Q38: The molecular geometry of the BeCl<sub>2</sub> molecule
Q54: Possible shapes of AB<sub>3</sub> molecules are linear,trigonal
Q69: An antibonding π orbital contains a maximum
Q108: Nitrogen is colorless because the minimum energy
Q151: The 1s hydrogen orbital overlaps with the
Q157: There is/are _ π bond(s)in the molecule
Q158: Each molecular orbital can accommodate, at most,
Q160: The hybrid orbitals used for bonding by