Multiple Choice
The hybridization of the oxygen atom labeled y in the structure below is ________. The C-O-H bond angle is ________. 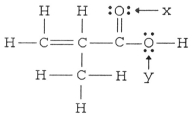
A) sp, 180°
B) sp2, 109.5°
C) sp3, 109.5°
D) sp3d2, 90°
E) sp, 90°
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: There are _ σ and _ π
Q19: A molecule has the formula AB<sub>3</sub> and
Q24: The molecular geometry of the left-most carbon
Q25: Of the following species,_ will have bond
Q27: The sensation of vision results from a
Q28: The Lewis structure of carbon monoxide is
Q46: The hybrid orbital set used by the
Q52: The central atom in a certain molecule
Q53: The electron-domain geometry and the molecular geometry
Q95: Using the VSEPR model,the electron-domain geometry of