Multiple Choice
The Lewis structure of carbon dioxide is given below. The hybridization of the carbon atom in carbon dioxide is ________. 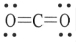
A) sp3
B) sp2
C) sp
D) sp2d
E) sp2d2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: For molecules of the general formula AB<sub>n</sub>,n
Q34: Electrons in core orbitals contribute to atom
Q44: According to MO theory,overlap of two s
Q70: In a typical multiple bond, the σ
Q77: The order of MO energies in B<sub>2</sub>,
Q80: Of the following, only _ appears to
Q114: The molecular geometry of the BrO<sub>3</sub><sup>-</sup> ion
Q116: A molecular orbital can accommodate a maximum
Q124: The molecular geometry of the PF<sub>4</sub><sup>+</sup> ion
Q148: The hybrid orbital set used by the