Multiple Choice
There is/are ________ σ bond(s) in the molecule below. 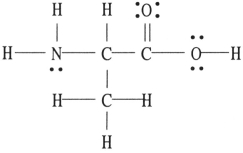
A) 1
B) 2
C) 12
D) 13
E) 18
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Of the molecules below,only _ is polar.<br>A)CCl<sub>4</sub><br>B)CH<sub>4</sub><br>C)SeF<sub>4</sub><br>D)SiCl<sub>4</sub>
Q29: The highest energy occupied molecular orbital in
Q37: Using the VSEPR model,the molecular geometry of
Q57: The hybridizations of nitrogen in NF<sub>3</sub> and
Q74: Using the VSEPR model,the electron-domain geometry of
Q76: The electron-domain geometry and molecular geometry of
Q82: The hybridization of the carbon atom in
Q105: The angles between sp<sup>2</sup> orbitals are _.<br>A)45°<br>B)180°<br>C)90°<br>D)109.5°<br>E)120°
Q131: The F-B-F bond angle in the BF<sub>2</sub><sup>-</sup>
Q163: The electron-domain geometry of the AsF<sub>6</sub><sup>-</sup> ion