Multiple Choice
There is/are ________ π bond(s) in the molecule below. 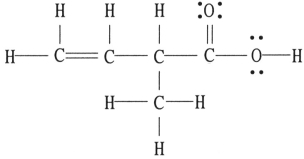
A) 0
B) 1
C) 2
D) 4
E) 16
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: XeF<sub>4</sub> is a polar molecule.
Q11: A typical double bond consists of _.<br>A)three
Q51: For a molecule with the formula AB<sub>2</sub>,the
Q85: In a polyatomic molecule, "localized" bonding electrons
Q88: According to MO theory,overlap of two p
Q92: ClF<sub>3</sub> has "T-shaped" geometry. There is/are _
Q103: Based on molecular orbital theory,the bond order
Q133: The H-C-H bond angle in the CH<sub>4</sub>
Q146: Using the VSEPR model,the molecular geometry of
Q155: The molecular geometry of the SiH<sub>2</sub>Cl<sub>2</sub> molecule