Multiple Choice
In ideal gas equation calculations, expressing pressure in Pascals (Pa) , necessitates the use of the gas constant, R, equal to ________.
A) 0.08206 atm L mol-1K-1
B) 8.314 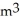 -Pa/mol-K
-Pa/mol-K
C) 62.36 L torr mol-1K-1
D) 1.987 cal mol-1K-1
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: CO (5.00 g)and CO<sub>2</sub> (5.00 g)were placed
Q17: A sample of gas (24.2 g)initially at
Q37: A sample of a gas (5.0 mol)at
Q127: The effusion rate of a gas is
Q141: Using the van der Waals equation,the pressure
Q142: The average kinetic energy of the particles
Q144: In a Torricelli barometer, a pressure of
Q145: The volume of HCl gas required to
Q145: The density of chlorine (Cl<sub>2</sub>)gas at 25
Q168: How many molecules are there in 4.00