Multiple Choice
Of the following, only ________ is impossible for an ideal gas.
A) 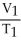 =
= 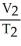
B) 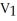
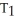 =
= 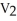
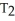
C) 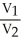 =
= 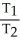
D) 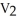 =
= 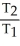
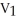
E) 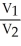 =
= 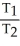 = 0
= 0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: CO (5.00 g)and CO<sub>2</sub> (5.00 g)were placed
Q94: A balloon originally had a volume of
Q115: CO (5.00 g)and CO<sub>2</sub> (5.00 g)were placed
Q127: The effusion rate of a gas is
Q129: The reaction of 50 mL of Cl<sub>2</sub>
Q130: A vessel contains a mixture of 34.9
Q131: The molecular weight of a gas that
Q132: A mixture of two gases was allowed
Q145: The volume of HCl gas required to
Q168: How many molecules are there in 4.00