Multiple Choice
A gas vessel is attached to an open-end manometer filled with a nonvolatile liquid of density 0.993 g/mL as shown below. 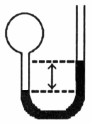 The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is ________ atm.
The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is ________ atm.
A) 1.05
B) 1.01
C) 0.976
D) 0.993
E) 1.08
Correct Answer:

Verified
Correct Answer:
Verified
Q96: Two deviations of real gases from ideal
Q107: The reaction of 25 mL of N<sub>2</sub>
Q110: The rms speed of methane molecules at
Q111: The density of N<sub>2</sub>O at 1.53 atm
Q113: A sample of a gas (1.50 mol)is
Q114: The pressure exerted by a column of
Q116: The density of chlorine gas at 1.01
Q117: The reaction of 50 mL of N<sub>2</sub>
Q119: Of the following gases,_ will have the
Q149: Standard temperature and pressure (STP),in the context