Multiple Choice
Using the van der Waals equation, the pressure in a 22.4 L vessel containing 1.00 mol of neon gas at 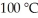 is ________ atm. (a = 0.211 L2-atm/mol2, b = 0.0171 L/mol)
is ________ atm. (a = 0.211 L2-atm/mol2, b = 0.0171 L/mol)
A) 0.730
B) 1.00
C) 1.21
D) 1.37
E) 0.367
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q47: A sample of He gas (2.0 mmol)effused
Q47: The volume of fluorine gas required to
Q49: A vessel containing Ne(g)and Ar(g)has a total
Q50: A 0.333-g sample of an unknown pure
Q52: In a gas mixture of He, Ne,
Q55: The molecular weight of a gas that
Q58: A pressure of 0.500 atm is the
Q73: According to the kinetic-molecular theory,molecules of different
Q91: A gas originally at 27 °C and
Q144: Ammonium nitrite undergoes thermal decomposition to produce