Multiple Choice
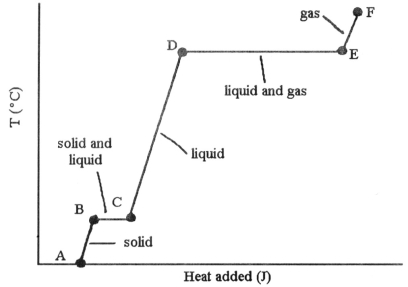
-The phase changes B → C and D → E are not associated with temperature increases because the heat energy is used up to ________.
A) increase distances between molecules
B) break intramolecular bonds
C) rearrange atoms within molecules
D) increase the velocity of molecules
E) increase the density of the sample
Correct Answer:

Verified
Correct Answer:
Verified
Q44: As a gaseous element condenses,the atoms become
Q46: What is the predominant intermolecular force in
Q50: Boron triiodide (BI<sub>3</sub>)melts at 49.9 °C and
Q53: A _ liquid crystal has the least
Q54: In liquids,the attractive intermolecular forces are _.<br>A)very
Q56: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2701/.jpg" alt=" -On the phase
Q58: Which of the following characteristics would prevent
Q59: What type(s)of intermolecular forces exist between Br<sub>2</sub>
Q103: What intermolecular force is responsible for the
Q118: How high a liquid will rise up