Multiple Choice
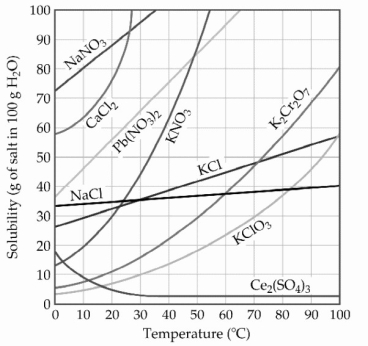
-A sample of potassium nitrate (49.0 g) is dissolved in 101 g of water at 100 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and no precipitate is observed. This solution is ________.
A) hydrated
B) placated
C) saturated
D) unsaturated
E) supersaturated
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The concentration (M)of HCl in a solution
Q3: The vapor pressure of pure water at
Q29: Emulsifying agents typically have a hydrophobic end
Q47: Which of the following substances is more
Q53: A solution is prepared by adding 40.00
Q56: Calculate the molality of a 10.0% (by
Q60: What is the molality of sodium chloride
Q62: A solution containing 20.0 g of an
Q72: As the concentration of a solute in
Q158: Which of the following will have an