Multiple Choice
The data in the table below were obtained for the reaction:
A + B → P 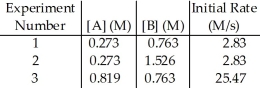
-The magnitude of the rate constant is ________.
A) 38.0
B) 0.278
C) 13.2
D) 42.0
E) 2.21
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: The rate constant of a first-order process
Q22: Which of the following is true?<br>A)If we
Q37: The mechanism for formation of the product
Q87: As the temperature of a reaction is
Q90: The active site of nitrogenase is a
Q92: The relationship of absorbed light to the
Q93: A compound decomposes by a first-order process.
Q95: The rate constant of a first-order process
Q96: Elementary reactions involving the simultaneous collision of
Q120: In the energy profile of a reaction,the