Multiple Choice
Which of the following expressions is the correct equilibrium-constant expression for the following reaction? CO2 (g) + 2H2 (g)  CH3OH (g)
CH3OH (g)
A) 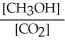
B) 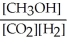
C) 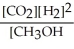
D) 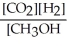
E) 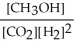
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q79: The effect of a catalyst on a
Q80: Given the following reaction at equilibrium, if
Q81: In which of the following reactions would
Q82: Consider the following reaction at equilibrium: 2CO<sub>2</sub>
Q83: Phosphorous trichloride and phosphorous pentachloride equilibrate in
Q85: Consider the following reaction at equilibrium: C
Q86: At 400 K, the equilibrium constant for
Q87: At 24° C, K<sub>p</sub> = 0.080 for
Q88: The K<sub>p</sub> for the reaction below is
Q89: The equilibrium constant (K<sub>p</sub>)for the interconversion of