Multiple Choice
The equilibrium expression for Kp for the reaction below is ________. 2O3 (g)  3O2 (g)
3O2 (g)
A) 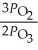
B) 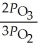
C) 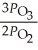
D) 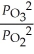
E) 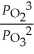
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q34: The K<sub>eq</sub> for the equilibrium below is
Q35: The value of K<sub>eq</sub> for the equilibrium
Q36: At 900.0 K, the equilibrium constant (K<sub>p</sub>)for
Q37: At 200 °C, the equilibrium constant (K<sub>p</sub>)for
Q38: If a reaction is endothermic, _ the
Q40: Which of the following expressions is the
Q41: The value of K<sub>eq</sub> for the equilibrium
Q42: The expression for K<sub>p</sub> for the reaction
Q43: For an exothermic reaction, increasing the reaction
Q44: A reaction vessel is charged with hydrogen