Multiple Choice
The equilibrium expression for Kp for the reaction below is ________. N2 (g) + O2 (g)  2NO (g)
2NO (g)
A) 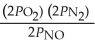
B) 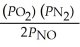
C) 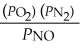
D) 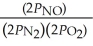
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q62: The value of K<sub>eq</sub> for the equilibrium
Q63: The equilibrium constant for the gas phase
Q64: The reaction below is exothermic: 2SO<sub>2</sub> (g)
Q65: The equilibrium constant for reaction 1 is
Q66: For the endothermic reaction CaCO<sub>3</sub> (s) <img
Q68: If the reaction quotient Q for a
Q69: In the coal-gasification process, carbon monoxide is
Q70: In which of the following reactions would
Q71: The expression of K<sub>eq</sub> for the following
Q72: At equilibrium,_.<br>A)all chemical reactions have ceased<br>B)the rates