Multiple Choice
Using the data in the table, which of the conjugate bases below is the strongest base? 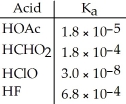
A) OAc-
B) CHO2-
C) ClO-
D) F-
E) OAc- and CHO2-
Correct Answer:

Verified
Correct Answer:
Verified
Q7: The pH of a 0.55 M aqueous
Q8: A 0.15 M aqueous solution of the
Q9: A 0.15 M aqueous solution of the
Q10: The conjugate acid of CH<sub>3</sub>NH<sub>2</sub> is _.<br>A)CH<sub>3</sub>NH<sub>2</sub><br>B)CH<sub>3</sub>NH<sub>3</sub><sup>+</sup><br>C)CH<sub>3</sub>NH<sub>2</sub><sup>+</sup><br>D)CH<sub>3</sub>NH<sup>+</sup><br>E)none
Q11: The acid-dissociation constants of sulfurous acid (H<sub>2</sub>SO<sub>3</sub>)are
Q13: Classify the following compounds as weak acids
Q17: A 0.1 M solution of _ has
Q87: Classify the following compounds as weak acids
Q108: The pH of a 0.25 M aqueous
Q136: Of the following,_ is a weak acid.<br>A)HF<br>B)HCl<br>C)HBr<br>D)HNO<sub>3</sub><br>E)HClO<sub>4</sub>