Multiple Choice
Using the data in the table, which of the conjugate bases below is the weakest base? 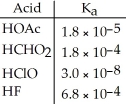
A) OAc-
B) CHO2-
C) ClO-
D) F-
E) OAc- and CHO2-
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: The magnitude of K<sub>w</sub> indicates that _.<br>A)water
Q24: Which of the following ions will act
Q38: An acid containing the COOH group is
Q86: Determine the pOH of a 0.10 M
Q87: The acid-dissociation constant at 25.0 °C for
Q89: A solution of acetic acid is 2.0%
Q90: A- is a weak base. Which equilibrium
Q91: A 1.0 × 10<sup>-2</sup> M aqueous solution
Q92: In which of the following aqueous solutions
Q93: The pH of a 0.30 M solution