Multiple Choice
Using the data in the table, which of the conjugate acids below is the strongest acid? 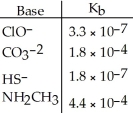
A) HClO
B) HCO3-
C) H2S
D) NH3CH3+
E) H2S and HClO
Correct Answer:

Verified
Correct Answer:
Verified
Q39: The conjugate base of H<sub>2</sub>PO<sub>4</sub><sup>-</sup> is _.<br>A)H<sub>3</sub>PO<sub>4</sub><br>B)HPO<sub>4</sub><sup>2-</sup><br>C)PO<sub>4</sub><sup>3-</sup><br>D)H<sub>3</sub>O<sup>+</sup><br>E)OH<sup>-</sup>
Q48: Determine the pH of a 0.15 M
Q49: What is the pOH of an aqueous
Q50: A 0.1 M aqueous solution of _
Q51: The K<sub>a</sub> of hypochlorous acid (HClO)is 3.0
Q52: Classify the following compounds as weak acids
Q54: Using the data in the table, which
Q55: Calculate the pOH of a 0.0727 M
Q56: Classify the following compounds as weak bases
Q57: The hydride ion, H<sup>-</sup>, is a stronger