Multiple Choice
Using the data in the table, which of the conjugate acids below is the weakest acid? 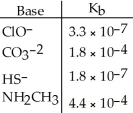
A) HClO
B) HCO3-
C) H2S
D) NH3CH3+
E) H2S and HClO
Correct Answer:

Verified
Correct Answer:
Verified
Q27: According to the Arrhenius concept,an acid is
Q63: Ammonia is a _.<br>A)weak acid<br>B)strong base<br>C)weak base<br>D)strong
Q111: Calculate the pH of a solution at
Q112: The base-dissociation constant, K<sub>b</sub>, for pyridine, C<sub>5</sub>H<sub>5</sub>N,
Q113: The K<sub>a</sub> of hypochlorous acid (HClO)is 3.0
Q114: The pH of an aqueous solution at
Q115: Calculate the concentration (in M)of hydroxide ions
Q118: What is the conjugate acid of NH<sub>3</sub>?<br>A)NH<sub>3</sub><br>B)NH<sub>2</sub><sup>+</sup><br>C)NH<sub>3</sub><sup>+</sup><br>D)NH<sub>4</sub><sup>+</sup><br>E)NH<sub>4</sub>OH
Q119: K<sub>a</sub> for HCN is 4.9 × 10<sup>-10</sup>.
Q133: HZ is a weak acid.An aqueous solution