Multiple Choice
Using the data in the table, which of the conjugate acids below is the strongest acid? 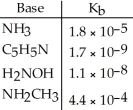
A) NH4+
B) C5H5NH+
C) H3NOH+
D) NH3CH3+
E) NH4+ and NH3CH3+
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q44: The pH of a 0.60 M aqueous
Q46: The molar concentration of hydronium ion in
Q61: What is the pH of an aqueous
Q62: Calculate the molarity of hydroxide ion in
Q63: The K<sub>a</sub> of acetic acid (HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>)is 1.8
Q64: Determine the pH of a 0.35 M
Q65: What is the pOH of a 0.030
Q67: What is the pH of an aqueous
Q68: A solution of ammonia is 2.0% ionized
Q69: The K<sub>a</sub> of hydrofluoric acid (HF)at 25.0