Multiple Choice
Using the data in the table, which of the conjugate acids below is the weakest acid? 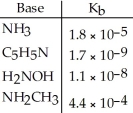
A) NH4+
B) C5H5NH+
C) H3NOH+
D) NH3CH3+
E) NH4+ and NH3CH3+
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: Which one of the following is a
Q33: What is the concentration (in M)of hydronium
Q36: Classify the following compounds as weak bases
Q37: What is the pH of an aqueous
Q39: What is the pH of an aqueous
Q41: What is the conjugate acid of CO<sub>3</sub><sup>2</sup><sup>-
Q42: A substance that is capable of acting
Q43: What is the pH of a 0.020
Q105: A Br∅nsted-Lowry acid is defined as a
Q126: Which of the following acids will be