Multiple Choice
Given the thermodynamic data in the table below, calculate the equilibrium constant (at 298 K) for the reaction: 2 SO2 (g) + O2 (g)  2 SO3 (g)
2 SO3 (g) 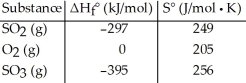
A) 2.40 × 1024
B) 1.06
C) 1.95
D) 3.82 × 1023
E) More data are needed.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: The value of ΔH° for the oxidation
Q40: The value of ΔH° for the decomposition
Q60: The value of ΔG° at 25 °C
Q63: The normal boiling point of water is
Q72: A reaction that is not spontaneous at
Q75: Find the temperature (in K)above which a
Q77: For a given reaction, ΔH = -24.2
Q78: The value of ΔG° (kJ/mol)at 25 °C
Q79: The standard Gibbs free energy of formation
Q85: A reaction that is spontaneous as written