Multiple Choice
Consider the reaction: FeO (s) + Fe (s) + O2(g) → Fe2O3 (s)
Given the following table of thermodynamic data at 298 K: 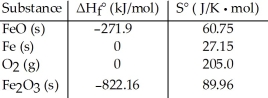 The value K for the reaction at 25 °C is ________.
The value K for the reaction at 25 °C is ________.
A) 370
B) 5.9 × 104
C) 3.8 × 10-14
D) 7.1 × 1085
E) 8.1 × 1019
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: The entropy of a pure crystalline substance
Q4: The normal boiling point of ethanol (C<sub>2</sub>H<sub>5</sub>OH)is
Q5: ΔS is positive for the reaction _.<br>A)2
Q8: Given the following table of thermodynamic data,
Q10: Of the following, the entropy of _
Q32: The value of ΔS° for the oxidation
Q35: Which reaction produces a decrease in the
Q58: The value of ΔG° at 25 °C
Q103: The second law of thermodynamics states that
Q107: Consider a pure crystalline solid that is