Multiple Choice
Of the following, the entropy of gaseous ________ is the largest at 25 °C and 1 atm.
A) C3H8
B) C3H6
C) C3H4
D) C2H6
E) 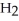
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: The value of ΔH° for the decomposition
Q20: Calculate ΔG°<sup> </sup>(in kJ/mol)for the following reaction
Q44: The standard Gibbs free energy of formation
Q56: The value of ΔG° at 25 °C
Q65: ΔS is positive for the reaction _.<br>A)Pb(NO<sub>3</sub>)<sub>2</sub>
Q79: The standard Gibbs free energy of formation
Q85: Which reaction produces a decrease in the
Q88: Which one of the following is always
Q89: The equilibrium constant for the following reaction
Q122: The combustion of acetylene in the presence