Multiple Choice
Table 20.2 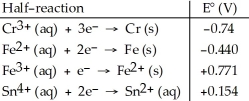
-Which of the following reactions will occur spontaneously as written?
A) Sn4+ (aq) + Fe3+ (aq) → Sn2+ (aq) + Fe2+ (aq)
B) 3Fe (s) + 2Cr3+ (aq) → 2Cr (s) + 3Fe2+ (aq)
C) Sn4+ (aq) + Fe2+ (aq) → Sn2+ (aq) + Fe (s)
D) 3Sn4+ (aq) + 2Cr (s) → 2Cr3+ (aq) + 3Sn2+ (aq)
E) 3Fe2+ (aq) → Fe (s) + 2Fe3+ (aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q5: How many minutes will it take to
Q12: The standard emf for the cell using
Q15: The standard cell potential (E°<sub>cell</sub>)for the voltaic
Q15: At constant temperature and pressure the Gibbs
Q22: In a voltaic cell,electrons flow from the
Q41: 1V = _.<br>A)1 amp ∙ s<br>B)1 J/s<br>C)96485
Q47: Consider an electrochemical cell based on the
Q56: Which transformation could take place at the
Q82: What is the oxidation number of manganese
Q109: The purpose of the salt bridge in