Multiple Choice
The half-reaction occurring at the cathode in the balanced reaction shown below is ________. 3MnO4- (aq) + 24H+ (aq) + 5Fe (s) → 3Mn2+ (aq) + 5Fe3+ (aq) + 12H2O (l)
A) MnO4- (aq) + 8H+ (aq) + 5 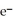 → Mn2+ (aq) + 4H2O (l)
→ Mn2+ (aq) + 4H2O (l)
B) 2MnO4- (aq) + 12H+ (aq) + 6e- → 2Mn2+ (aq) + 3H2O (l)
C) Fe (s) → Fe3+ (aq) + 3e-
D) Fe (s) → Fe2+ (aq) + 2e-
E) Fe2+ (aq) → Fe3+ (aq) + e-
Correct Answer:

Verified
Correct Answer:
Verified
Q19: _ is the reducing agent in the
Q49: What is the correct coefficient for the
Q63: Which one of the following reactions is
Q79: Which one of the following is the
Q86: Which of the following reactions is a
Q87: The most difficult species to reduce and
Q89: The standard cell potential (E°)of a voltaic
Q90: The standard cell potential (E° cell)for the
Q94: The balanced half-reaction in which dichromate ion
Q95: A voltaic cell is constructed with two