Multiple Choice
The relationship between the change in Gibbs free energy and the emf of an electrochemical cell is given by ________.
A) ΔG = 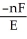
B) ΔG = 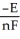
C) ΔG = -nFE
D) ΔG = -nRTF
E) ΔG = 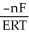
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: What is the cathode in an alkaline
Q49: What is the correct coefficient for the
Q92: What is the coefficient of the dichromate
Q95: A voltaic cell is constructed with two
Q98: The balanced half-reaction in which chlorine gas
Q101: What is the oxidation number of potassium
Q102: Which element is reduced in the reaction
Q103: The dependence of cell emf on concentration
Q104: How many minutes will it take to
Q105: What is the oxidation number of manganese