Multiple Choice
The missing product in this reaction combines with oxygen to form a compound with the formula ________. 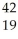 K →
K → 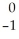 e + _____
e + _____
A) M2O
B) MO
C) MO2
D) M2O3
E) M3O2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: The mass of a proton is 1.00728
Q6: This reaction is an example of _.
Q7: <sup>210</sup>Pb has a half-life of 22.3 years
Q9: The nuclear disintegration series of _ is
Q10: High speed electrons emitted by an unstable
Q11: What isotope of what element is produced
Q84: When ionizing radiation enters the body,what is
Q113: Electrons do not exist in the nucleus,yet
Q151: In what type of radioactive decay does
Q161: All atoms of a given element have