Multiple Choice
What volume (L) of 0.250 M HNO3 is required to neutralize a solution prepared by dissolving 17.5 g of NaOH in 350 mL of water?
A) 50.0
B) 0.44
C) 1.75
D) 0.070
E) 1.75 × 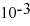
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q38: The point in a titration at which
Q56: Mixing 10.00 mL of an aqueous solution
Q79: An aqueous ethanol solution (400 mL)was diluted
Q84: Calculate the concentration (M)of sodium ions in
Q84: The net ionic equation for the dissolution
Q86: How many milliliters of a stock solution
Q87: How many moles of Co<sup>2+</sup> are present
Q91: What are the respective concentrations (M)of Cu<sup>+2</sup>
Q92: How many grams of H<sub>3</sub>PO<sub>4</sub> are in
Q112: How many moles of K<sup>+</sup> are present