Multiple Choice
The molarity (M) of an aqueous solution containing 22.5 g of sucrose (C12H22O11) in 35.5 mL of solution is __________.
A) 0.0657
B) 1.85 × 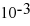
C) 1.85
D) 3.52
E) 0.104
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: How many grams of H<sub>3</sub>PO<sub>4</sub> are in
Q12: What mass (g)of AgBr is formed when
Q16: Which ion(s)is/are spectator ions in the formation
Q19: The spectator ions in the reaction between
Q34: The reaction between strontium hydroxide and chloric
Q63: Which one of the following compounds is
Q72: A 25.5 mL aliquot of HCl (aq)of
Q131: Which one of the following substances is
Q166: Which of the following reactions will not
Q166: A 0.100 M solution of _ will