Multiple Choice
Which electron configuration represents a violation of the Pauli exclusion principle?
A) 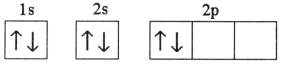
B) 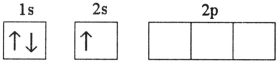
C) 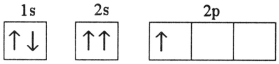
D) 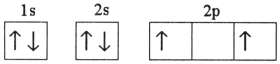
E) 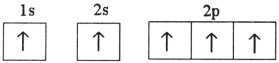
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q24: The wavelength of a photon that has
Q50: The de Broglie wavelength of a particle
Q58: The energy of a photon that has
Q101: The _ subshell contains only one orbital.<br>A)5d<br>B)6f<br>C)4s<br>D)3d<br>E)1p
Q108: The de Broglie wavelength of a car
Q141: Calculate the energy (J)change associated with an
Q148: Electromagnetic radiation with a wavelength of 525
Q149: The complete electron configuration of argon, element
Q167: Ham radio operators often broadcast on the
Q168: The largest principal quantum number in the