Multiple Choice
Which electron configuration represents a violation of the Pauli exclusion principle?
A) 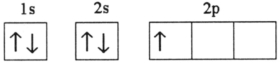
B) 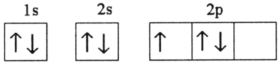
C) 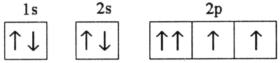
D) 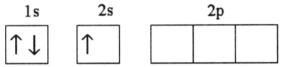
E) 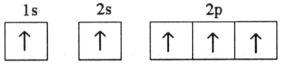
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q111: The larger the principal quantum number of
Q113: Which of the subshells below do not
Q128: The wavelength of light emitted from a
Q130: What is the frequency (s<sup>-1</sup>)of a photon
Q131: Which group in the periodic table contains
Q132: At maximum, an f-subshell can hold _
Q134: The de Broglie wavelength of an electron
Q135: The ground state electron configuration of Fe
Q136: The wavelength of light that has a
Q137: What is the frequency (s<sup>-1</sup>)of electromagnetic radiation